In today’s technologically-advanced world, there’s a tremendous opportunity for medical device innovators to translate their concepts into prototypes capable of having a profound impact on the future of healthcare.
Cast Silicone Medical Device Saves Oreo the Goat
This Spring, RapidMade teamed up with New York-based nonprofit Woodstock Farm Sanctuary for a special project to help a goat named Oreo deal with a unique medical condition.
As reported by the Shawangunk Journal, Woodstock Farm Sanctuary rescued Oreo from a petting zoo in 2015 where she had been neglected. After three happy years at Woodstock, Oreo was hospitalized in 2018 because of a buildup of abdominal fluid.
The issue flared up again this year, and veterinarians found that a tumor was preventing her stomach from functioning properly. When they recommend euthanasia for Oreo, however, Woodstock director Hervé Breuil refused to give up on her.
To help Oreo, Woodstock Farm Sanctuary recruited Dr. Isabelle Louge to develop a valve that would allow fluid to be released from Oreo’s abdomen. The only problem was that such a valve would need to be cast from rubber, which, using traditional technologies, would have been prohibitively expensive and time consuming to manufacture.
Cast Silicone with 3D Printing Tooling
RapidMade was able to help Oreo by deploying a combination of 3D printed tooling and rapid silicone casting. Instead of creating traditional tooling for the injection molding process, we used our Multi Jet Fusion 3D printer to quickly create a 3D printed master pattern. We then used this pattern to create a silicone mold that let us to cast a valve for Oreo in medical-grade silicone.
Not only did this allow us to deliver the valve in a fraction of the time and cost of injection molding, but it also made it possible for Woodstock Farm Sanctuary to create multiple iterations of the valve in a timely manner. Ultimately, we were able to create a product that met Oreo’s needs and was durable enough to stand up to the rough-and-tumble life of a goat.
Quickly Produce High-Quality Cast Rubber and Plastic Parts
Silicone and urethane casting are great options for small- to medium-volume productions that require the versatile materials and finish quality of injection molding. By combining these technologies with 3D printed master patterns, we can create high-quality plastic and rubber parts faster that would otherwise be possible and at less cost. This can benefit clients seeking to produce end-use parts or to create prototypes and first articles during their product development.
If you have a project that you think could benefit from 3D printing or cast silicone, reach out to us to learn more about our services or get started right away with a free quote and project analysis.
3D Printed Titanium Vertebrae Saves Life of Cancer Patient
Credit: 3Dprint.com
Dr. Ralph Mobbs of the Sydney Spine Clinic turned to 3D printing to save the life of a patient suffering from a rare form of cancer. Drage Josevski was diagnosed with chordoma, a cancer that affects the spine. His case was especially difficult because the tumor was located in his top two vertebrae. Dr. Mobbs performed a landmark procedure that replaced the vertebrae with a 3D printed titanium implant. Josevski’s surgery was a success, and he is in rehabilitation to adjust to the implant. This achievement is yet another example of the possibilities 3D printing creates for the medical field.
Son Helps Father Get a 3D Printed Face
Reconstructive Scientist scans son's face to 3D print a prosthesis for his dad (Photo Credit: 3Dprint.com)
Anyone who has experienced facial scarring can appreciate the pain and embarrassment that often accompanies the disfigurement, no matter how minor. After four relatively simple surgeries to reduce the scarring and improve the shape of my nose, I am still sensitive about how it looks - but then I read the following story in 3dprint.com which described the medical miracle of a man who got a new face - and lease on life - through 3D printing. To say it put things into perspective would be an understatement...
“Every year, thousands of individuals are left with terrible deformities due to their courageous battles against cancer. This was the case for a 74-year-old man named Keith Londsdale who had started his battle again cancer all the way back in 1990, undergoing 45 different procedures to save his life against a very aggressive form of basal cell carcinoma.
When all was said and done Londsdale’s life was spared. However, he was left without an upper jaw bone, cheekbones, his nose, and his palate, and in their place was a gaping hole. Without the ability to properly speak, eat or drink, doctors sought out a solution to make this brave man’s life as normal as possible.”
Until recently, most prostheses have been functionally or cosmetically lacking. (I remember a patient who had lost her lower jaw to disease, and she had a basic plastic cup that just sat where her jaw had been). Now, 3D scanning, modeling and printing are achieving lifelike results that closely match the recipient's existing features.
Keith Londsdale is one such beneficiary of medical additive manufacturing. His son, Scott, worked with Jason Watson, a Reconstructive Scientist at Nottinghams' Queen's Medical Center, to create a prosthetic that incorporated Scott's features to ensure a familial likeness.
“Watson had Scott come into the hospital where they 3D scanned his face. From the scans, a sophisticated computer algorithm created a 3D printable model, which the team at Queen’s Medical Centre was able to print out. Basically doctors now had a 3D printed physical replica of a portion of Scott’s face they then were able to copy in wax and create a mold from. From that mold they then created a silcone mask from Scott, which fit Keith’s face nearly perfectly.”
Imagine the day when such prostheses are bio printed using living skin cells.
Could 3D Scanning and Printing Improve Splint Production and Fit?
This week, I graduated from a soft cast to a hand splint, custom made from low-temperature thermoplastic and Velcro. The process was an interesting mix of art and science. My Occupational Therapist, who specializes in hand injuries, regularly creates them "while you wait."
The procedure began with a gross sizing using a paper pattern placed against my arm to determine how much thermoplastic material would be needed. After the two sheets were cut down, each was heated in a hot water bath and then carefully molded against my arm to obtain a form fit - a snug, protective shield whose edges roll away from the skin to prevent chafing. Later the two splints were joined by a series of strategically placed Velcro strips. Unfortunately, I encountered pinch points which prompted a return visit this morning... alas, as I type this post, I suspect there will be more tweaking needed.
So I find myself debating whether the fitting process could be improved using a 3D scanner and printer. Theoretically, a doctor's staff could quickly scan the patient's hand in the office to create a model that could then be used to make the splints. The final fitting would then be completed during the first OT appointment, allowing the patient to begin therapy during the initial visit instead.
But would the additive manufacturing approach be much more accurate than the current in-office process? I suspect it might be more expensive (but I won't know until I get the bill) and since the splints are only worn (hopefully) for about four weeks, insurance companies might not approve. But given how tender the injured body part can be, anything that minimizes the amount of handling required during the fitting would be welcomed.
If the 3D Screw Fits, Wear It
Screws and plate used to repair a hip fracture (image credit 3Dprint.com
I wish RapidMade's recent blogging silence was due to the holidays. Alas, I was sidelined by a bad fall down a steep flight of stairs. Shortly after I broke my foot, shattered my wrist, and learned I needed surgery, I remembered a conversation I had with an NIH representative at the FDA's meeting on 3D printing. He expressed frustration that, despite additive manufacturing being more widely adopted in medicine, many procedures were not benefitting from its customization. Ironically, he specifically mentioned the screws used in orthopedic surgery, saying it was frightening that patients' bones were modified to accommodate the screws and not vise versa.
I was in too much pain to think to ask my surgeon if my standard-issued screws and plate matched my bones well enough or ask how often fit is a problem, so I will have to speculate on what factors have slowed its adoption.
First, I suspect screws could quickly and easily be cut to fit. But if that is true then why would surgeons ever alter the recipient's bones instead?
I also wonder if the simple screw and plate designs make 3D printing them more expensive and time consuming than traditional manufacturing, especially if custom fittings are rarely required. Interestingly, I just read about a hip surgery where Dr. Bagaria repaired a hip fracture by taking CT scans to create a 3D print that allowed him to plan the surgery and customize his approach.
“Using the model, Dr. Bagaria was able to create a 7-hole reconstruction plate that was pre-contured. They then used the model to carry out a surgical simulation prior to taking part in the real thing. The surgeons were able to drill the screw trajectories, measure the screw lengths required, and confirm the positions of the plate, all with the help of the model” (3Dprint.com).”
Perhaps a major reason 3D printed screws aren't in great demand is that surgeons don't often have CT scans of the broken bones and are therefore less likely to know fit will be an issue until the patient is on the operating table. Honestly, fixing most broken bones is fairly straight foward - and truthfully the xrays were painful enough, I don't know if I'd have welcomed getting CT scans as well. Having said this, I was told my wrist was worse than expected, requiring more work than expected, so who knows?
I won't know my outcome until Monday when the cast comes off, but I'm guessing screw size won't be a problem. I'll just be happy to type with two hands.
FDA Considers Approach to Additive Manufacturing of Medical Devices
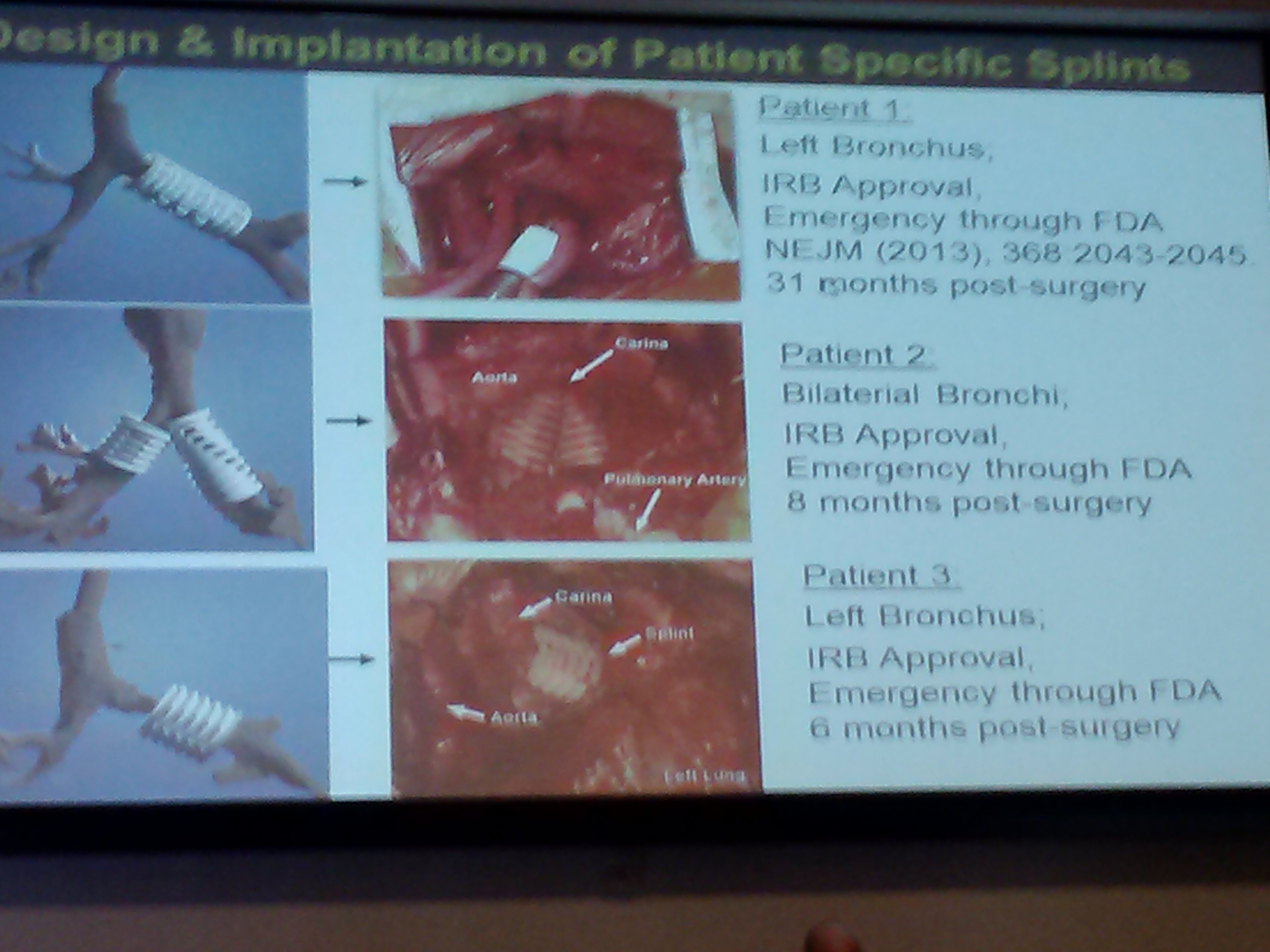
Patient-specific printed splints are used to treat life-threatening thoracic constrictions. Work done at the University of Michigan involves laser sintering bio compatible, bio absorbable materials.
The official purpose of this week's FDA-sponsored workshop was "to provide a forum for FDA, medical device manufacturers, additive manufacturing companies and academia to discuss technical challenges and solutions of 3D printing." In other words, the FDA wants "input to help it determine technical assessments that should be considered for additively manufactured devices to provide a transparent evaluation process for future submissions."
The FDA is trying to stay current with advanced manufacturing technologies that are revolutionizing patient care and, in some cases, democratizing its availability... When a next-door neighbor can print a medical device in his or her basement, that clearly has many positive and negative implications that need to be considered.
Ignoring the regulatory implications for a moment (I'll get to those shortly), the presentations were fascinating. In particular, I was intrigued and inspired by the Post-Printing speakers and Clinical Perspectives projects.
STERIS representative Dr. Brodbeck cautioned that the complex designs and materials now being created with additive manufacturing make sterilization practices challenging. How will the manufacturer know if the implant is sterile or if the agent has been adequately removed or if it is suitable? Some materials and designs, for example, cannot tolerate acids, heat or pressure.
Wake Forest Presenter Dr. Yoo shares his institution's research on bioprinting
Dr Boland from the University of Texas El Paso shared his team's work on 3D printed tissues. Using inkjet technology, the researchers are evaluating the variables involved in successfully printing skin. Another bio-printing project being undertaken at Wake Forest by Dr. Yoo involves constructing bladder-shaped prints using bladder cell biopsies and scaffolding. And Dr. Liacouras at Walter Reed discussed his institution's practice of using 3D printing to create surgical guides and custom implants.
Since RapidMade creates anatomical models, one project, near and dear to my heart - pun intended - is work done at Children's National Hospital by Drs. Krieger and Olivieri. The physicians use printed cardiac models to "inform clinical decisions" ie. evaluate conditions, plan surgeries, and reduce operating time.
As interesting as the presentations were, the subsequent discussions were arguably more important. In an attempt to identify and address all significant impacts of additive manufacturing on medical device production, the subject was organized into pre-printing (input), printing (process) and post-printing (output) considerations. Panelists and other stakeholders shared their concerns and viewpoints on each topic in an attempt to inform and persuade FDA decision makers.
An interesting (but expected) outcome was the relative positions of the various stakeholders. Well establish and large manufacturers proposed validation procedures: material testing, process operating guidelines, quality control, traceability programs, etc. Independent makers argued that this approach would impede, if not eliminate, their ability to provide low-cost prosthetic devices.
Coming from the highly regulated food industry, I completely understand and accept the need to adopt similar measures for some additively manufactured medical devices. An implant is going into someone's body, so the manufacturer needs to evaluate and assure the quality of raw materials, processing procedures and finished product. But this means, as in the food industry, the manufacturer needs to know the composition of materials. Suppliers cannot hide behind proprietary formulations. If manufacturers are expected to certify that a device is safe, they need to know what ingredients are in the materials they are using.
Hopefully, the FDA will also agree with the GE representative who suggested that manufacturers should be expected to certify the components and not the process. What matters is whether or not the device is safe, not what process was used to make it. Another distinction should be the product's risk level. Devices should continue to be classified as I, II or III and that classification, not the process used, should determine its level of regulation.
If you are interested in submitting comments to the FDA on this topic, email them to http://www.regulations.gov .
3D Printers Promote Healing Tissues and Bones
PrintAlive BioPrinter Process...
Image Credit: Inside 3DP
Researchers at the University of Toronto have built the PrintAlive Bioprinter which prints skin grafts derived from a host patient's own skin cells. These cells, used as the material "ink" needed to produce the build, are deposited into strips that contain fewer cells than are typical in the "full continuous sheets" commonly used. The benefits of this approach are two-fold: it is faster than using cultured skin cells which take two weeks or more to grow enough to be grafted. And when skin damage runs deeper than the epidermis, this technique's bioprint pattern allows multiple layers to be applied and still survive.
The team includes Masters students Arianna Mcallister and Lian Lend, PhD student Boyang Zhang and University of Toronto Associate Professor of Mechanical and Industrial Engineering Axel Guenther. To date, their research has been confined to mice, but the researchers reportthe technology has worked to heal "severe wounds" and they expect human trials may be possible in two to three years.
Further south, a research team at the University of Massachusetts Medical school, led by Dr. Jie Song, is using a MakerBot Replicator to print a latticed scaffold implant it hopes will someday promote healing in damaged bones and tissues. Unlike the traditional filaments used in FDM printers, this 3D printer is fed a combination of "plastic and the therapeutic stem cells or proteins that a patient needs to heal, and the flexible scaffold that emerges could become a kind of patch for use by surgeons." The lab is also investigating a similar approach to "regenerate the periosteum, a tissue that covers bone."