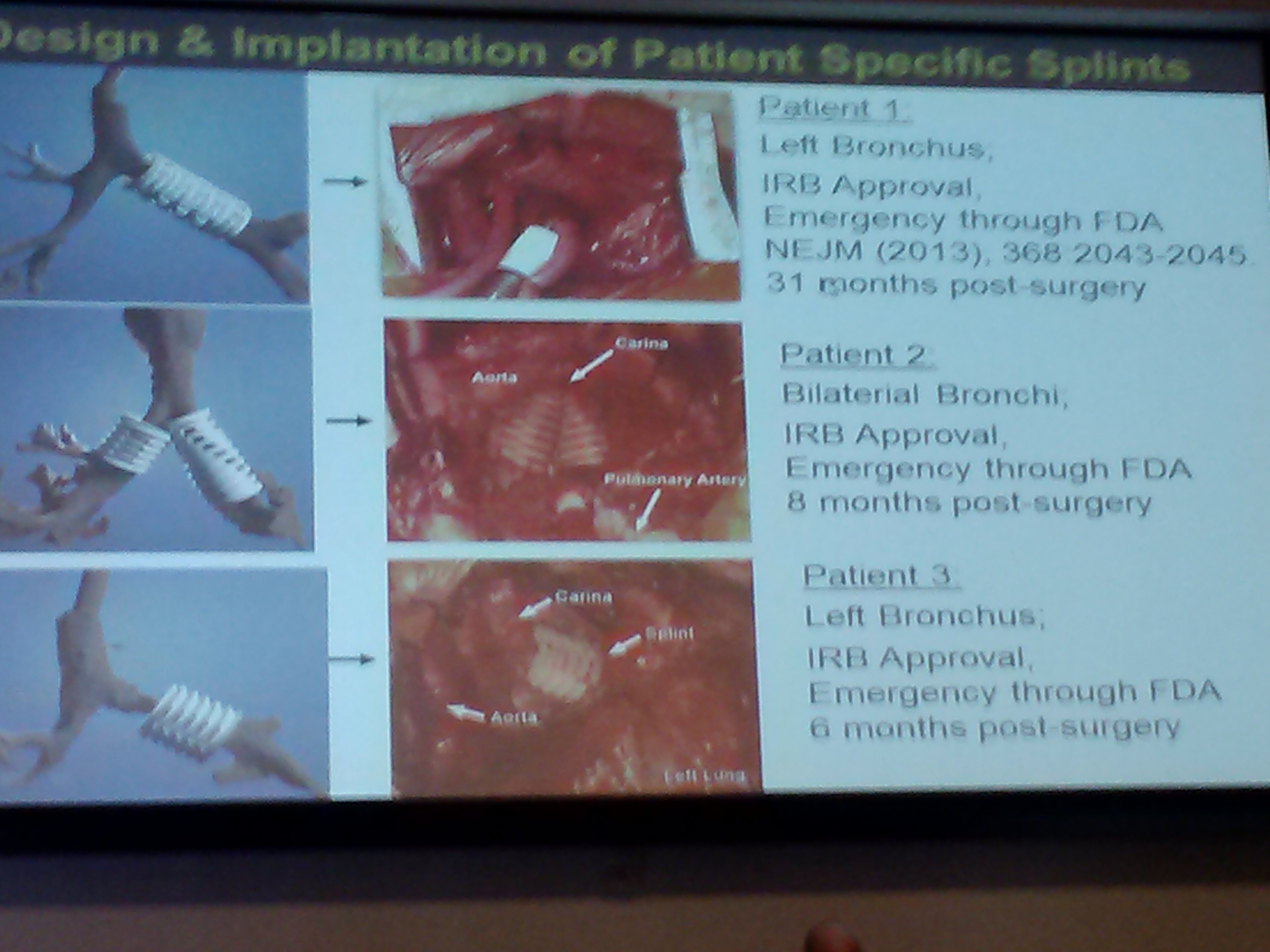
Dr Boland from the University of Texas El Paso shared his team's work on 3D printed tissues. Using inkjet technology, the researchers are evaluating the variables involved in successfully printing skin. Another bio-printing project being undertaken at Wake Forest by Dr. Yoo involves constructing bladder-shaped prints using bladder cell biopsies and scaffolding. And Dr. Liacouras at Walter Reed discussed his institution's practice of using 3D printing to create surgical guides and custom implants.
Since RapidMade creates anatomical models, one project, near and dear to my heart - pun intended - is work done at Children's National Hospital by Drs. Krieger and Olivieri. The physicians use printed cardiac models to "inform clinical decisions" ie. evaluate conditions, plan surgeries, and reduce operating time.
As interesting as the presentations were, the subsequent discussions were arguably more important. In an attempt to identify and address all significant impacts of additive manufacturing on medical device production, the subject was organized into pre-printing (input), printing (process) and post-printing (output) considerations. Panelists and other stakeholders shared their concerns and viewpoints on each topic in an attempt to inform and persuade FDA decision makers.
An interesting (but expected) outcome was the relative positions of the various stakeholders. Well establish and large manufacturers proposed validation procedures: material testing, process operating guidelines, quality control, traceability programs, etc. Independent makers argued that this approach would impede, if not eliminate, their ability to provide low-cost prosthetic devices.
Coming from the highly regulated food industry, I completely understand and accept the need to adopt similar measures for some additively manufactured medical devices. An implant is going into someone's body, so the manufacturer needs to evaluate and assure the quality of raw materials, processing procedures and finished product. But this means, as in the food industry, the manufacturer needs to know the composition of materials. Suppliers cannot hide behind proprietary formulations. If manufacturers are expected to certify that a device is safe, they need to know what ingredients are in the materials they are using.
Hopefully, the FDA will also agree with the GE representative who suggested that manufacturers should be expected to certify the components and not the process. What matters is whether or not the device is safe, not what process was used to make it. Another distinction should be the product's risk level. Devices should continue to be classified as I, II or III and that classification, not the process used, should determine its level of regulation.
If you are interested in submitting comments to the FDA on this topic, email them to http://www.regulations.gov .